With decades of hands-on experience across 88 rare disease indications—including support of more than 240 projects in the past 5 years—Premier Research offers comprehensive guidance to successfully advance your global rare disease drug development program from initial concept to market realization.
Our integrated services span new product assessment, regulatory strategy & consulting, nonclinical services and full-service clinical trial solutions, all tailored to meet the unique challenges of rare diseases.
Why Choose Premier?
- Deep Therapeutic Experience: Proven experience in rare disease and rare oncology, including support of 10 orphan drug approvals in the last 5 years.
- Focused Regulatory Guidance: Strong relationships with global regulators help us to achieve orphan drug designation and streamline approval timelines.
- Robust Feasibility: Data-driven approaches and long-standing relationships ensure early feasibility insights support optimized site selection and operational success.
- Innovative Program Design: Expert consultancy in alternative study designs—essential when recruiting hard-to-reach populations, or when placebo or comparator arms are not possible.
- Optimized Patient Engagement: Rapid recruitment powered by our state-of-the-art partnerships with biodatabanks, advocacy groups and KOLs.
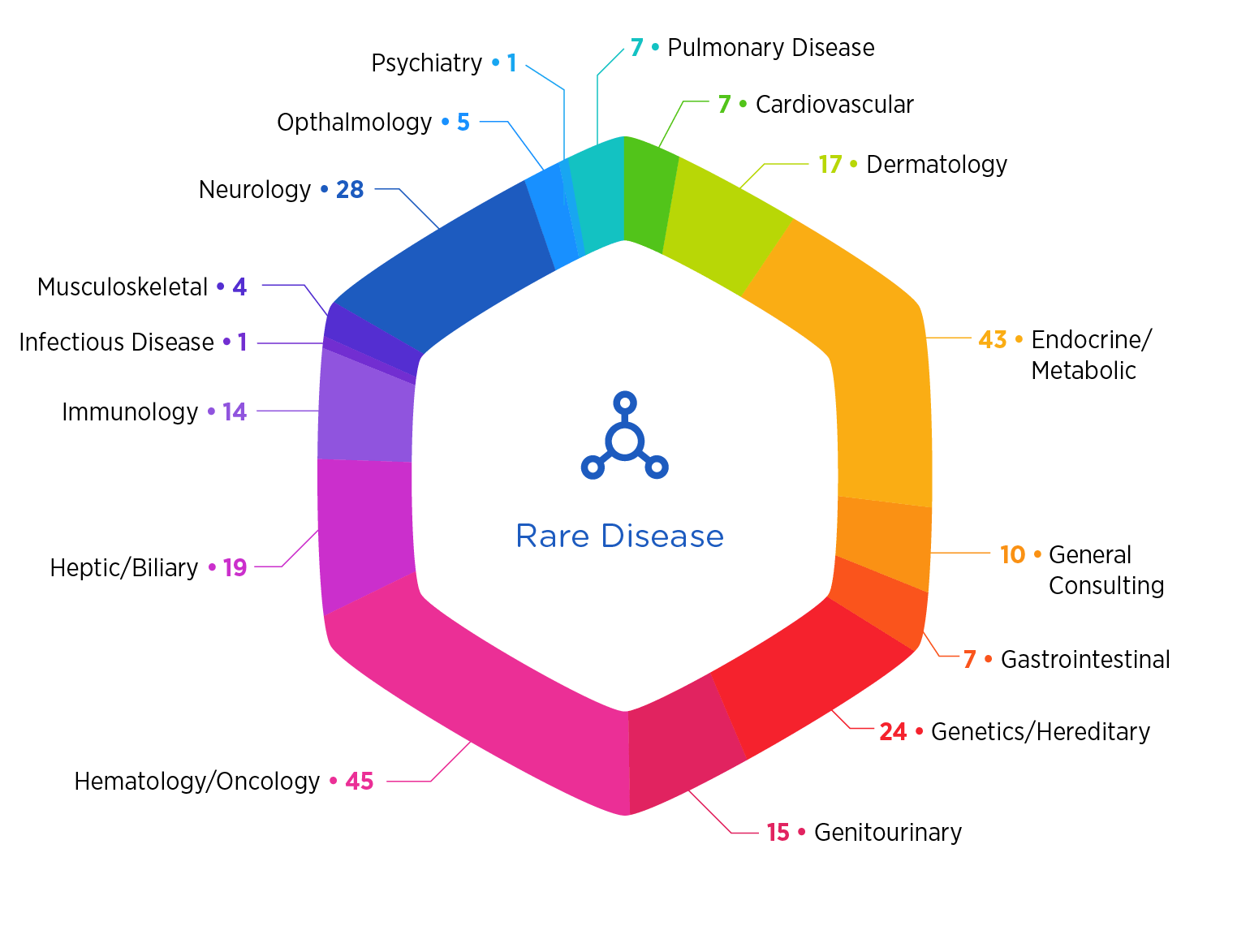
Your Partner in Rare Disease Development
With dedicated rare disease experts bringing together scientific depth, operational excellence, and a holistic approach to product development, Premier Research is your partner for advancing your rare disease development program. Whether you're navigating regulatory hurdles, designing adaptive trials, or identifying the right patients, our rare disease team is here to guide your program every step of the way.
Download our checklist, 9 Best Practices to Optimize Clinical Trial Efficiency and Quality, today or contact us to discuss your program.